what does lialh4 reduce Why does lialh4 reduce esters, amides, or carboxylic acids, while nabh4
Hey there! Today, let’s dive into the fascinating world of organic chemistry. We’ll be exploring the concept of reducing agents, with a specific focus on Lithium Aluminum Hydride (LiAlH4). Get ready for some mind-blowing reactions and transformations!
Reducing Agent Reactions with LiAlH4
Lithium Aluminum Hydride (LiAlH4) is a powerful reducing agent commonly used in organic chemistry. It has the incredible ability to convert functional groups within organic molecules, resulting in significant changes in their chemical properties and structures.
 One of the most remarkable transformations achieved through LiAlH4 is the reduction of carboxylic acids. These compounds, commonly found in nature and extensively used in various industries, can be converted into primary alcohols using this powerful reducing agent.
One of the most remarkable transformations achieved through LiAlH4 is the reduction of carboxylic acids. These compounds, commonly found in nature and extensively used in various industries, can be converted into primary alcohols using this powerful reducing agent.
 Imagine the possibilities that such a transformation can bring! From a simple carboxylic acid, which typically exhibits acidic properties, LiAlH4 can help us obtain a primary alcohol with its unique set of chemical characteristics. This opens up new avenues in pharmaceutical, chemical, and biological research, as these primary alcohols can be further modified to synthesize a range of valuable compounds.
Imagine the possibilities that such a transformation can bring! From a simple carboxylic acid, which typically exhibits acidic properties, LiAlH4 can help us obtain a primary alcohol with its unique set of chemical characteristics. This opens up new avenues in pharmaceutical, chemical, and biological research, as these primary alcohols can be further modified to synthesize a range of valuable compounds.
Furthermore, the reduction of carboxylic acids is just one example of the numerous reactions LiAlH4 is involved in. It also exhibits the ability to reduce aldehydes, ketones, esters, and many other functional groups. Each reduction reaction brings us closer to a deeper understanding of the various organic compounds and their potential applications.
The power of LiAlH4 lies in its ability to act as a “super” hydrogen donor. By transferring hydride ions (H-) to the organic molecule, it effectively reduces the molecule by adding hydrogen atoms. This reduction process alters the chemical structure and properties of the starting compound, leading to the formation of new compounds with diverse functionalities.
As we continue to explore the capabilities of reducing agents like LiAlH4, we uncover new pathways and solutions in the field of organic chemistry. The ability to selectively modify specific functional groups within a molecule offers exciting prospects for drug discovery, materials science, and various other scientific endeavors.
So, the next time you come across the term “reducing agent,” remember the incredible potential it holds, especially with the mighty LiAlH4 at its disposal. Organic chemistry continues to amaze us with its endless possibilities, and LiAlH4 is one of its unsung heroes!
Keep discovering and experimenting, fellow chemistry enthusiasts!
If you are searching about Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid you’ve visit to the right place. We have 5 Pics about Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid like Does LiAlH4 reduce Amides class 11 chemistry CBSE, Mécanisme de réduction des carbonyles LiAlH4 et NaBH4 | Mefics and also Mécanisme de réduction des carbonyles LiAlH4 et NaBH4 | Mefics. Here you go:
Lithium Aluminum Hydride (LiAlH4) For Reduction Of Carboxylic Acid
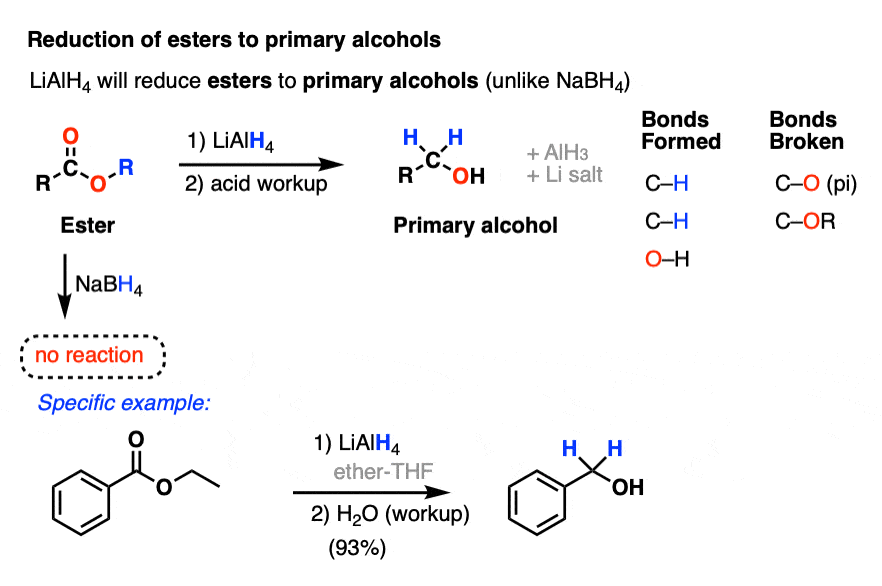
 www.masterorganicchemistry.comReducing Agent Reactions–LiAlH4 | Organic Chemistry, Chemistry
www.masterorganicchemistry.comReducing Agent Reactions–LiAlH4 | Organic Chemistry, Chemistry
 www.pinterest.comlialh4 lah lithium hydride nabh4 mechanism ester reactions riduzione mechanisms composti litio organici chemia lialh nh2 rxns hydrierung amide organica
www.pinterest.comlialh4 lah lithium hydride nabh4 mechanism ester reactions riduzione mechanisms composti litio organici chemia lialh nh2 rxns hydrierung amide organica
Does LiAlH4 Reduce Amides Class 11 Chemistry CBSE
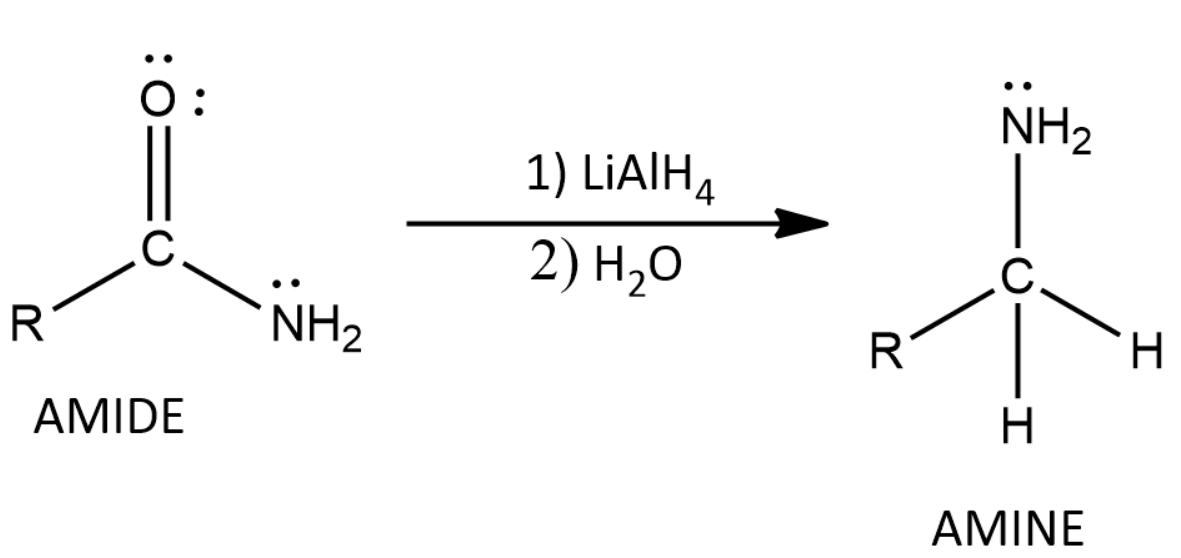 www.vedantu.comlialh4 amides lial reducing
www.vedantu.comlialh4 amides lial reducing
Why Does LiAlH4 Reduce Esters, Amides, Or Carboxylic Acids, While NaBH4
www.quora.comnabh4 lialh4 carboxylic acids esters amides cannot chimie
Mécanisme De Réduction Des Carbonyles LiAlH4 Et NaBH4 | Mefics
 mefics.orgDoes lialh4 reduce amides class 11 chemistry cbse. Mécanisme de réduction des carbonyles lialh4 et nabh4. Why does lialh4 reduce esters, amides, or carboxylic acids, while nabh4
mefics.orgDoes lialh4 reduce amides class 11 chemistry cbse. Mécanisme de réduction des carbonyles lialh4 et nabh4. Why does lialh4 reduce esters, amides, or carboxylic acids, while nabh4